On April 24, 2025, a team led by Professor Guangshuo Ou from the School of Life Sciences at Tsinghua University published a major study in the Journal of Cell Biology titled “Phosphorylation-dependent regional motility of the ciliary kinesin OSM-3”. The research uncovers a precise phosphorylation-based mechanism regulating the regional activation and inactivation of OSM-3, a key kinesin motor protein involved in intracellular transport and ciliogenesis.
🧠 Why This Matters
Kinesins are ATP-powered motor proteins essential for microtubule-based intracellular transport, including the construction and maintenance of cilia. Previous studies have shown that dysregulation of kinesin activity is associated with neurodegenerative diseases, but the spatiotemporal control of kinesin function within cells has remained elusive—until now.
This new research identifies a phosphorylation-dependent switch that controls where and when the ciliary kinesin OSM-3 becomes active.
🔬 Key Findings
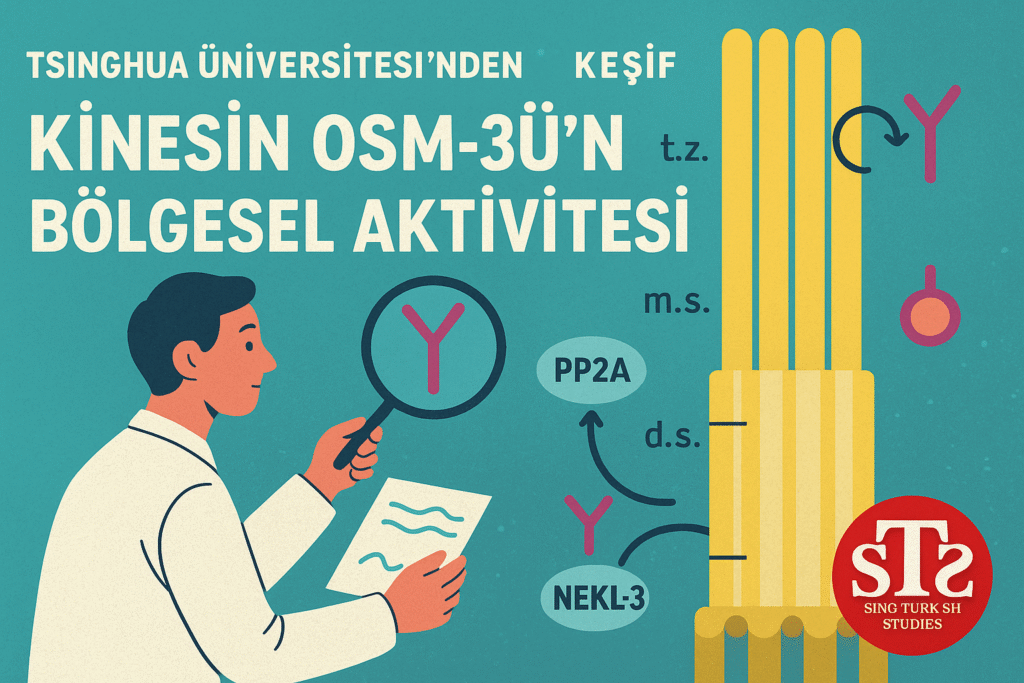
Professor Ou’s team investigated the interplay between NEK family kinases (specifically NEKL-3) and the PP2A phosphatase in modulating OSM-3’s activity during ciliogenesis:
- NEKL-3 kinase directly phosphorylates the motor domain of OSM-3, suppressing its ATPase activity and molecular motility.
- PP2A phosphatase counteracts this inhibition. Overexpression of PP2A prematurely activates OSM-3, while its absence reduces activity and shortens cilia.
- Despite being in an extended (active-like) conformation at the ciliary base, OSM-3 remains functionally inactive until it reaches its designated segment of the cilium.
- These effects were confirmed using single-molecule assays, mass spectrometry, and fluorescence lifetime imaging microscopy (FLIM).
This regulation ensures that Kinesin-II and OSM-3 operate sequentially and region-specifically during intraflagellar transport—Kinesin-II in the middle segment, OSM-3 in the distal segment—preserving functional compartmentalization of the cilium.
📈 Implications for Molecular Motor Research
This discovery offers a new framework for understanding kinesin regulation, and potentially extends to other kinesin family members due to the conserved nature of NEK kinases and PP2A phosphatases. The findings could open new pathways for studying motor protein dysfunction in diseases and designing targeted molecular therapies.
🧪 Article Information and Research Support
📄 Article Title: Phosphorylation-dependent regional motility of the ciliary kinesin OSM-3
🧬 Published in: Journal of Cell Biology, April 24, 2025
🔗 DOI: https://doi.org/10.1083/jcb.202407152
🧑🔬 Lead Author: Prof. Guangshuo Ou
👨🔬 Support: Cryo-EM Platform at Tsinghua University
🎓 Funding:
- Beijing Frontier Research Center for Biological Structure
- Tsinghua-Peking Center for Life Sciences
- Ministry of Science and Technology of China
- National Natural Science Foundation of China
Keywords:
- kinesin phosphorylation mechanism
- OSM-3 regulation
- ciliary kinesin activity
- kinesin and neurodegeneration
- NEKL-3 kinase OSM-3
- PP2A phosphatase kinesin
- kinesin motor protein control
- molecular motor regulation
- Tsinghua kinesin research
- intraflagellar transport regulation
New study from Tsinghua reveals how phosphorylation controls kinesin OSM-3 activity in cilia, offering insight into molecular motor regulation and ciliogenesis.